Laboratory Guidance
These are excerpts from a larger interim document provided by the World Health Organization, and should be used for informational/educational purposes only. FOR FULL GUIDANCE ON HOW TO COLLECT, SHIP, AND TEST 2019-nCoV PLEASE REFER TO THE ENTIRE DOCUMENT: WHO Guidance on Laboratory Testing for 2019-nCoV in suspected human cases
As information about the etiology, clinical manifestations and transmission of disease in the cluster of respiratory disease patients identified in Wuhan is evolving, the WHO will continue to monitor developments and revise recommendations as necessary.
The etiologic agent responsible for the cluster of pneumonia cases in Wuhan has been identified as a novel betacoronavirus (in the same family as SARS-CoV and MERS-CoV) via next generation sequencing (NGS) from cultured virus or directly from samples received from several pneumonia patients. Electron microscopy revealed a virus with a characteristic crown morphology, hence the name a coronavirus.
Full genome sequence data from the viruses have been shared officially with WHO and on the GISAID platform (https://www.gisaid.org/) and can inform the development of specific diagnostic tests for this emergent coronavirus. It is expected that validated PCR tests will become available soon. Until that time, the goals of diagnostic testing are to detect conventional causes of pneumonia early, to support disease control activities, and to work with reference laboratories that can perform pan coronavirus detection and directed sequencing.
Rapid collection and testing of appropriate specimens from suspected cases is a priority and should be guided by a laboratory expert. As extensive testing is still needed to confirm the 2019-nCoV and the role of mixed infection has not been verified, multiple tests may need to be performed and sampling sufficient clinical material is recommended. Local guidelines should be followed regarding patient or guardian’s informed consent for specimen collection, testing and potentially future research.
Specimens to be collected from symptomatic patients:
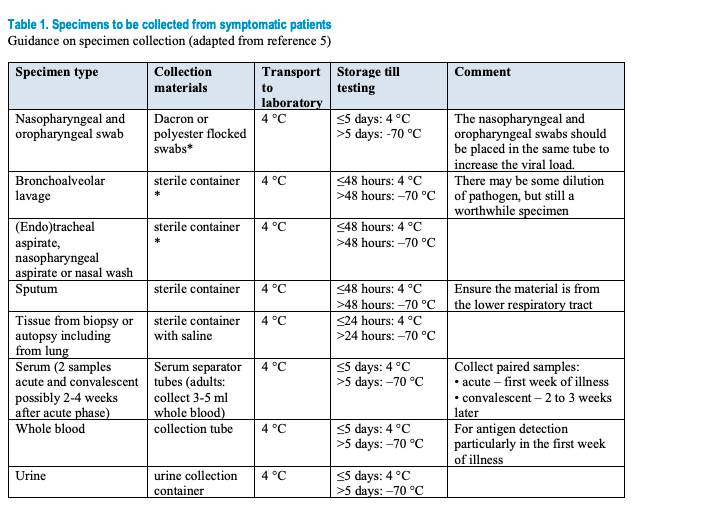
Safety procedures during sample collection and transport All specimens collected for laboratory investigations should be regarded as potentially infectious, and HCWs who collect, or transport clinical specimens should adhere rigorously to infection prevention and control guidelines and national or international regulations for the transport of dangerous goods (infectious substances) to minimize the possibility of exposure to pathogens. Implement the appropriate infection prevention and control precautions, guidance on IPC for the 2019-nCoV has been drafted.
2019-nCoV testing for patients that meet the suspected case definition Patients that meet the case definition for suspected 2019- nCoV should be screened for the virus with PCR (details below). If case management requires, screen also for other common causes of respiratory illness according to local guidelines (1,5,7). As coinfections can occur, all patients that meet the case definition should be tested for 2019-nCoV regardless of whether a conventional respiratory pathogen is found. If testing does not occur in an expert/reference laboratory it is encouraged to send the sample for confirmation to a regional, national or international reference laboratory with pan-coronavirus or specific 2019- nCoV detection capacity. WHO can assist Member States to identify laboratories able to provide this support.
Reporting of cases and test results Laboratories should follow national reporting requirements, but in general, suspected cases should be reported to relevant public health authorities as soon as the laboratory receives a specimen, even before any testing is performed. All test results, whether positive or negative, should likewise be immediately reported to national authorities. If the infection becomes widespread, laboratories should notify public health authorities immediately of each new confirmed case or positive screening test if there will be a delay in confirmatory testing. Laboratories should also periodically report the number of negative test results to public health.
Detection of a possible human case of emerging pathogen causing severe acute respiratory disease should immediately be notified to local, subnational and national public health authorities.
Additional resources:
For any concerns about your own symptoms, call your healthcare provider (UHS Advice Line for students at (510 643-7197). For more information on the University Health Services response, please refer to the UHS health advisory message.
